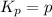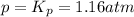Chemistry, 08.04.2020 01:53 cathydaves
The dissociation of calcium carbonate has an equilibrium constant of Kp = 1.16 at 1073 K . CaCO3(s) ⇄ CaO(s) + CO2(g) If you place 35.9 g of CaCO3 in a 9.56-L container at 1073 K, what is the pressure of CO2 in the container?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 03:50
Which of the following statements about acidic water is true? a. acid has no effect on the h,o molecules. b. the solution contains a larger number of oh ions than h,o ions. c. the solution contains a larger number of h,o ions than qh ions. d. the solution contains an equal number of h,o ions and oh ions. none of the above e.
Answers: 1

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
The dissociation of calcium carbonate has an equilibrium constant of Kp = 1.16 at 1073 K . CaCO3(s)...
Questions

Mathematics, 21.05.2021 05:30

Mathematics, 21.05.2021 05:30






Geography, 21.05.2021 05:30


Mathematics, 21.05.2021 05:30

Mathematics, 21.05.2021 05:30

English, 21.05.2021 05:30

Mathematics, 21.05.2021 05:30

Advanced Placement (AP), 21.05.2021 05:30

Mathematics, 21.05.2021 05:30



Mathematics, 21.05.2021 05:30


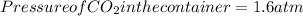
 ⇄
⇄ 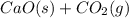
![K_p=[CO_2]](/tpl/images/0588/5116/7aeb1.png)
![[CO_2]=p](/tpl/images/0588/5116/a1554.png)