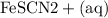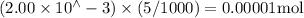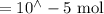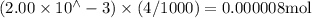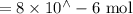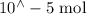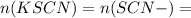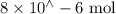Suppose a student mixes 4.00 mL of 2.00 # 10-3 M Fe(NO3 ) 3 with 5.00 mL of 2.00 # 10-3 M KSCN and 1.00 mL of water. The student then determines the [FeNCS2+] at equilibrium to be 8.75 # 10-5 M. Find the equilibrium constant for the following reaction. Show all your calculations for each step. Fe3+ (aq) + SCN- (aq) FeNCS2+ (aq) Step 1. Calculate the initial number of moles of Fe3+ and SCN- (use Equation 12). moles of Fe3+ moles of SCN- Step 2. How many moles of FeNCS2+ are present at equilibrium? What is the volume of the equilibrium mixture? mL moles of FeNCS2+ How many moles of Fe3+ and SCN- are consumed to produce the FeNCS2+? moles of Fe3+ moles of SCN-

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 01:00
Consider three unlabeled bottles, each contain small pieces of one of the following metals. - magnesium - sodium - silver the following reagents are used for identifying the metals. - pure water - a solution of 1.0 molar hcl - a solution of concentrated hno3 (a) which metal can be easily identified because it is much softer than the other two? describe a chemical test that distinguishes this metal from the other two, using only one of the reagents above. write a balanced chemical equation for the reaction that occurs. (b) one of the other two metals reacts readily with the hcl solution. identify the metal and write the balanced chemical equation for the reaction that occurs when this metal is added to the hcl solution. use the table of standard reduction potentials (attached) to account for the fact that this metal reacts with hcl while the other does not. (c) the one remaining metal reacts with the concentrated hno3 solution. write a balanced chemical equation for the reaction that occurs. (d) the solution obtained in (c) is diluted and a few drops of 1 m hcl is added. describe what would be observed. write a balanced chemical equation for the reaction that occurs.
Answers: 2

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1
You know the right answer?
Suppose a student mixes 4.00 mL of 2.00 # 10-3 M Fe(NO3 ) 3 with 5.00 mL of 2.00 # 10-3 M KSCN and 1...
Questions




English, 26.09.2019 20:40


History, 26.09.2019 20:40

World Languages, 26.09.2019 20:40


History, 26.09.2019 20:50

Mathematics, 26.09.2019 20:50


Mathematics, 26.09.2019 20:50


History, 26.09.2019 20:50





Social Studies, 26.09.2019 20:50

Social Studies, 26.09.2019 20:50

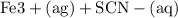 <---->
<---->