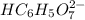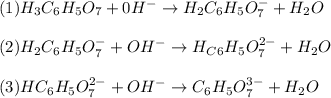Chemistry, 08.04.2020 01:44 jayline2003
: Citric acid, H3C6H5O7, is a triprotic acid. Consider a buffer system comprising H2C6H5O7 - and HC6H5O7 2- ions. What is the net ionic equation for the reaction that occurs when NaOH is added to a buffer containing H2C6H5O7 - and HC6H5O7 2- ?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1


Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1
You know the right answer?
: Citric acid, H3C6H5O7, is a triprotic acid. Consider a buffer system comprising H2C6H5O7 - and HC6...
Questions

English, 19.05.2021 18:20

Business, 19.05.2021 18:20


Mathematics, 19.05.2021 18:20

Spanish, 19.05.2021 18:20


English, 19.05.2021 18:20

English, 19.05.2021 18:20


Mathematics, 19.05.2021 18:20




Biology, 19.05.2021 18:20

Mathematics, 19.05.2021 18:20


Social Studies, 19.05.2021 18:20


Mathematics, 19.05.2021 18:20

Mathematics, 19.05.2021 18:20

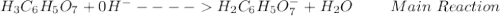
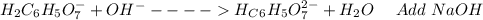
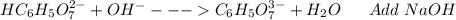
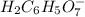 and
and