
While performing the Acid-catalyzed Hydrolysis of Epoxides experiment you used a solution containing 1.84 mL of cyclohexene oxide. After the reaction was completed and worked up, you obtained 0.739 g of 1,2-cyclohexane diol. Calculate the moles of starting material used.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 07:30
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1
You know the right answer?
While performing the Acid-catalyzed Hydrolysis of Epoxides experiment you used a solution containing...
Questions


Mathematics, 13.08.2020 21:01



Biology, 13.08.2020 21:01












History, 13.08.2020 21:01


Mathematics, 13.08.2020 21:01

English, 13.08.2020 21:01

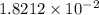 mole
mole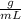
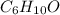
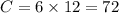
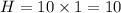
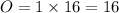
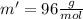
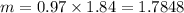
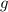
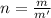
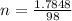
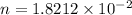 mol
mol

