
9. Diiodine pentoxide is useful in devices such as respirators because it reacts with the dangerous gas carbon monoxide, CO, to produce relatively harmless CO2 according to the following equation: I2O5 5CO £ I2 5CO2 a. In testing a respirator, 2.00 g of carbon monoxide gas is passed through diiodine pentoxide. Upon analyzing the results, it is found that 3.17 g of I2 was produced. Calculate the percentage yield of the reaction. b. Assuming that the yield in (a) resulted because some of the CO did not react, calculate the mass of CO that passed through.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 12:40
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

You know the right answer?
9. Diiodine pentoxide is useful in devices such as respirators because it reacts with the dangerous...
Questions


Mathematics, 14.07.2021 14:00

Chemistry, 14.07.2021 14:00

Mathematics, 14.07.2021 14:00



Mathematics, 14.07.2021 14:00

Social Studies, 14.07.2021 14:00

Chemistry, 14.07.2021 14:00

Mathematics, 14.07.2021 14:00

Arts, 14.07.2021 14:00

Mathematics, 14.07.2021 14:00


Chemistry, 14.07.2021 14:00

Mathematics, 14.07.2021 14:00


Mathematics, 14.07.2021 14:00

Mathematics, 14.07.2021 14:00


Mathematics, 14.07.2021 14:00

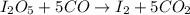
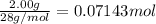
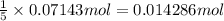 of iodine gas
of iodine gas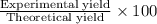
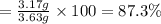
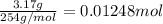
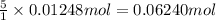 of carbon monoxide
of carbon monoxide

