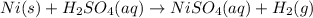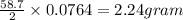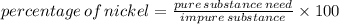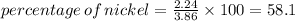Chemistry, 08.04.2020 01:38 dancemomsrule1
III. (4 points) A sample of nickel ore, which has nickel as the only metal present, is treated with an excess of sulfuric acid (H2SO4) to form nickel(II) sulfate and molecular hydrogen. (a) Write a balanced equation for the reaction. (b) If 0.0764 g of H2 is obtained from 3.86 g of the nickel ore, calculate the percent nickel, by mass, of the nickel ore.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2


Chemistry, 22.06.2019 04:00
Electric charge is what ? a. kinetic energy b. radiation c. discovery d. electricity
Answers: 1

Chemistry, 22.06.2019 05:50
What are the 4 phases of matter in order of increasing engery content?
Answers: 2
You know the right answer?
III. (4 points) A sample of nickel ore, which has nickel as the only metal present, is treated with...
Questions

English, 27.01.2021 23:10

Physics, 27.01.2021 23:10

Spanish, 27.01.2021 23:10


Physics, 27.01.2021 23:10

Biology, 27.01.2021 23:10

Mathematics, 27.01.2021 23:10





English, 27.01.2021 23:10


English, 27.01.2021 23:10



Mathematics, 27.01.2021 23:10


Mathematics, 27.01.2021 23:10