
Chemistry, 07.04.2020 23:59 ryliepeloquinf
A laser is emitting photons with a wavelength of 639.8 nm. What is the energy for 1 mole of these photons? For Planck's constant, use a value of 6.626x10-34 J s. Use units of kJ/mol. Report just the number, not the units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 12:30
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 15:00
Describe what happens to the molecules as water goes from ice to liquid to vapor. be sure to explain what happens to the temperature during the phase changes.
Answers: 2
You know the right answer?
A laser is emitting photons with a wavelength of 639.8 nm. What is the energy for 1 mole of these ph...
Questions

Mathematics, 05.05.2020 14:14



Geography, 05.05.2020 14:14


Mathematics, 05.05.2020 14:14

Mathematics, 05.05.2020 14:14



Mathematics, 05.05.2020 14:14

Mathematics, 05.05.2020 14:14



Mathematics, 05.05.2020 14:14

Mathematics, 05.05.2020 14:14

Mathematics, 05.05.2020 14:14

Chemistry, 05.05.2020 14:14


English, 05.05.2020 14:14

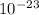
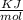
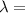 639.8 ×
639.8 × 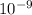 m
m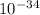 J sec
J sec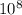 meter per second
meter per second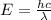
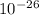
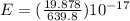
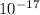 Joule per mole
Joule per mole

