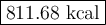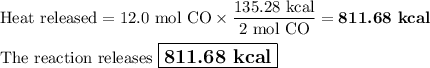Chemistry, 07.04.2020 23:54 hardwick744
Carbon monoxide reacts with oxygen to form carbon dioxide by the following reaction: 2 CO(g) + O2(g) → 2 CO2(g) ∆H for this reaction is −135.28 kcal. How much heat would be released if 12.0 moles of carbon monoxide reacted with sufficient oxygen to produce carbon dioxide?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 23.06.2019 08:30
Of element x has 22 protons, how many electrons does it have
Answers: 1
You know the right answer?
Carbon monoxide reacts with oxygen to form carbon dioxide by the following reaction: 2 CO(g) + O2(g)...
Questions



Mathematics, 22.11.2019 11:31

Mathematics, 22.11.2019 11:31


English, 22.11.2019 11:31


Mathematics, 22.11.2019 11:31

Mathematics, 22.11.2019 11:31

Mathematics, 22.11.2019 11:31

World Languages, 22.11.2019 11:31




Mathematics, 22.11.2019 11:31



History, 22.11.2019 11:31


Mathematics, 22.11.2019 11:31