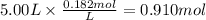The reaction 2N2O5 (g) → 2N2O4 (g) + O2 (g) has a reaction rate that is dependent only on the concentration of N2O5 and at a certain temperature has a rate constant k of 0.0168 s-1. If 2.50 moles of N2O5 were placed in a 5.00 liter container at that temperature, how many moles of N2O5 would remain after 1.00 min?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1

Chemistry, 23.06.2019 02:50
Select the correct location on the image identify the element that humans need to breathe. 2015 er r ights reserved
Answers: 3

Chemistry, 23.06.2019 11:00
The standard emf for the cell using the overall cell reaction below is +2.20 v: 2al(s) + 3i2(s) → 2ai3+(aq) + 6i-(aq) the emf generated by the cell when [ai3+] = 3.5 × 10-3 m and [i-] = 0.015 m is v. the standard emf for the cell using the overall cell reaction below is +2.20 v: 2al(s) + 3i2(s) 2ai3+(aq) + 6i-(aq) the emf generated by the cell when [ai3+] = 3.5 × 10-3 m and [i-] = 0.015 m is v. 2.36 2.24 2.21 2.51 2.04
Answers: 2
You know the right answer?
The reaction 2N2O5 (g) → 2N2O4 (g) + O2 (g) has a reaction rate that is dependent only on the concen...
Questions

Health, 26.09.2019 11:20

Mathematics, 26.09.2019 11:20


History, 26.09.2019 11:20

Physics, 26.09.2019 11:20

Mathematics, 26.09.2019 11:20

Biology, 26.09.2019 11:20




Mathematics, 26.09.2019 11:20

Mathematics, 26.09.2019 11:20

Business, 26.09.2019 11:20

History, 26.09.2019 11:20

Chemistry, 26.09.2019 11:20


World Languages, 26.09.2019 11:20


English, 26.09.2019 11:20

Mathematics, 26.09.2019 11:20

![[N_2O_5] = [N_2O_5]_0 \times e^{-k \times t}](/tpl/images/0587/8861/ec7f1.png)
![[N_2O_5]_0](/tpl/images/0587/8861/4a20d.png) : initial concentrationk: rate constantt: time
: initial concentrationk: rate constantt: time![[N_2O_5] = 0.500 M \times e^{-0.0168 s^{-1} \times 60s} = 0.182 M](/tpl/images/0587/8861/ce720.png)