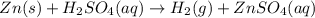Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is the ph of a solution with a concentration of 5.2 × 10–8 m h3o+?
Answers: 1


Chemistry, 23.06.2019 01:00
If a sample of radioactive isotopes takes 600 minutes to decay from 400 grams to 50 grams, what is the half-life of the isotope?
Answers: 1

Chemistry, 23.06.2019 06:30
How can the number of core electrons be determined from the periodic table
Answers: 1
You know the right answer?
Solid zinc metal reacts with sulfuric acid to form hydrogen gas and an aqueous solution of zinc (II)...
Questions


Mathematics, 20.03.2021 20:40

English, 20.03.2021 20:40

Arts, 20.03.2021 20:40


Mathematics, 20.03.2021 20:40

Mathematics, 20.03.2021 20:40

Mathematics, 20.03.2021 20:40

Mathematics, 20.03.2021 20:40

History, 20.03.2021 20:40

English, 20.03.2021 20:40


Mathematics, 20.03.2021 20:40

Mathematics, 20.03.2021 20:40

Mathematics, 20.03.2021 20:40



Advanced Placement (AP), 20.03.2021 20:40


Mathematics, 20.03.2021 20:40