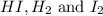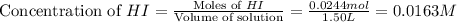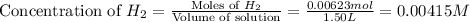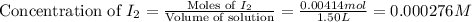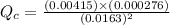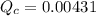Chemistry, 07.04.2020 23:09 mcckenziee
Consider the equilibrium system described by the chemical reaction below. Calculate the value of Qc for the initial set reaction conditions in a 1.50 L container: 0.00623 mol H₂, 0.00414 mol I₂, 0.0244 mol HI.\

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
Consider the equilibrium system described by the chemical reaction below. Calculate the value of Qc...
Questions






English, 20.12.2020 02:20

Computers and Technology, 20.12.2020 02:20







Mathematics, 20.12.2020 02:20

Mathematics, 20.12.2020 02:20


History, 20.12.2020 02:20


History, 20.12.2020 02:20

Mathematics, 20.12.2020 02:20

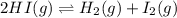
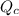 is, 0.00431
is, 0.00431![Q_c=\frac{[H_2][I_2]}{[HI]^2}](/tpl/images/0587/8224/7d2ae.png)