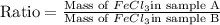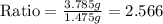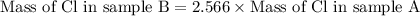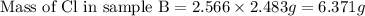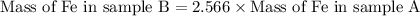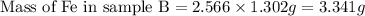Chemistry, 29.09.2019 17:30 hernandezbrandon059
Use the law of constant composition to complete the following table summarizing the amounts of iron and chlorine produced upon the decomposition of the sample of iron(iii) chloride. mass fecl3 mass fe mass cl sample a3.785 g1.302 g2.483 g sample b1.475 g

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 16:00
How will the volume of a gas be affected if the pressure is tripled, but the temperature remains the same?
Answers: 3

You know the right answer?
Use the law of constant composition to complete the following table summarizing the amounts of iron...
Questions

Mathematics, 07.04.2021 18:30

Mathematics, 07.04.2021 18:30




Mathematics, 07.04.2021 18:30

Arts, 07.04.2021 18:30

English, 07.04.2021 18:30


History, 07.04.2021 18:30

Physics, 07.04.2021 18:30


Advanced Placement (AP), 07.04.2021 18:30

Business, 07.04.2021 18:30

Mathematics, 07.04.2021 18:30


Mathematics, 07.04.2021 18:30


Mathematics, 07.04.2021 18:30

Mathematics, 07.04.2021 18:30

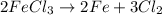
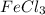 .
.