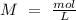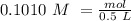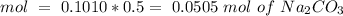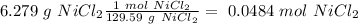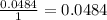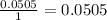Chemistry, 07.04.2020 21:28 danielahalesp87vj0
Determine the limiting reactant for the reaction of sodium carbonate and nickel(II) chloride using the quantities listed below. 6.279 g solid nickel(II) chloride 500.0 mL of 0.1010 M sodium carbonate

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 03:30
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1


Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
Determine the limiting reactant for the reaction of sodium carbonate and nickel(II) chloride using t...
Questions




Mathematics, 22.08.2019 14:30

Mathematics, 22.08.2019 14:30

English, 22.08.2019 14:30

History, 22.08.2019 14:30




Health, 22.08.2019 14:30

Biology, 22.08.2019 14:30

History, 22.08.2019 14:30



History, 22.08.2019 14:30



Mathematics, 22.08.2019 14:30

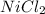
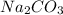 ) and the Nickel (II) Chloride (
) and the Nickel (II) Chloride (