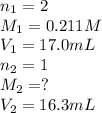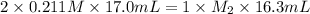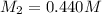Chemistry, 07.04.2020 21:20 jadbaubl1449
A titration reached the equivalence point when 17.0 mL of was added to of NaOH (aq) of unknown concentration. What is the concentration (M) of this unknown NaOH solution? H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + 2H2O(l)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Which form of relativism states that people rely on their own standards of right and wrong when making a decision?
Answers: 1

Chemistry, 22.06.2019 03:30
If you have 5.25 grams of methane (ch4), how many grams of co2 will you produce ?
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

You know the right answer?
A titration reached the equivalence point when 17.0 mL of was added to of NaOH (aq) of unknown conce...
Questions




Mathematics, 31.12.2019 02:31

History, 31.12.2019 02:31

History, 31.12.2019 02:31

History, 31.12.2019 02:31


Chemistry, 31.12.2019 02:31


Mathematics, 31.12.2019 02:31

Mathematics, 31.12.2019 02:31



Geography, 31.12.2019 02:31

Mathematics, 31.12.2019 02:31

Mathematics, 31.12.2019 02:31

Social Studies, 31.12.2019 02:31


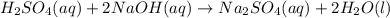
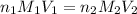
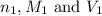 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 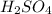
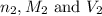 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.