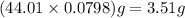Gaseous methane (CH4) reacts with gaseous oxygen (O2) gas to produce gaseous carbon dioxide (CO2) and gaseous water (H2O). What is the theoretical yield of carbon dioxide formed from the reaction of 1.28 g of methane and 10.1 g of oxygen gas? Be sure your answer has the correct number of significant digits in it.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 20:30
Water undergoes a large change in density at 0 ∘ c as it freezes to form ice. calculate the percent change in density that occurs when liquid water freezes to ice at 0 ∘ c given that
Answers: 2

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1
You know the right answer?
Gaseous methane (CH4) reacts with gaseous oxygen (O2) gas to produce gaseous carbon dioxide (CO2) an...
Questions

German, 22.06.2020 23:57

Mathematics, 22.06.2020 23:57

Health, 22.06.2020 23:57

Mathematics, 22.06.2020 23:57

Mathematics, 22.06.2020 23:57

Social Studies, 22.06.2020 23:57


Mathematics, 22.06.2020 23:57

Mathematics, 22.06.2020 23:57




History, 22.06.2020 23:57


German, 22.06.2020 23:57

Mathematics, 22.06.2020 23:57


Physics, 22.06.2020 23:57

Mathematics, 22.06.2020 23:57

Mathematics, 22.06.2020 23:57

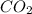 is 3.51 g.
is 3.51 g.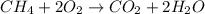
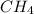 = 16.04 g/mol
= 16.04 g/mol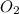 = 32.00 g/mol
= 32.00 g/mol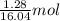 of
of 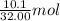 of
of