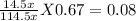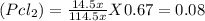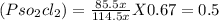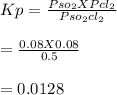Chemistry, 07.04.2020 20:02 dhgdzfbzdf6765
At 25°C, gaseous decomposes to and to the extent that 14.5% of the original (by moles) has decomposed to reach equilibrium. The total pressure (at equilibrium) is 0.670 atm. Calculate the value of for this system.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
At 25°C, gaseous decomposes to and to the extent that 14.5% of the original (by moles) has decompose...
Questions



English, 05.10.2019 08:00

Mathematics, 05.10.2019 08:00


Mathematics, 05.10.2019 08:00


Mathematics, 05.10.2019 08:00

Mathematics, 05.10.2019 08:00


Mathematics, 05.10.2019 08:00


Chemistry, 05.10.2019 08:00



History, 05.10.2019 08:00

Arts, 05.10.2019 08:00

Mathematics, 05.10.2019 08:00


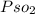 ) =
) =