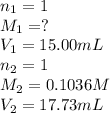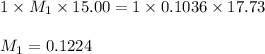Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 21.06.2019 23:00
An electrons position cannot be known precisely only it's probability of being in a certain location can be known
Answers: 1
You know the right answer?
7. A student titrated a 15.00-mL sample of a solution containing a weak, monoprotic acid with NaOH....
Questions



Social Studies, 07.01.2022 06:40


SAT, 07.01.2022 06:40

Social Studies, 07.01.2022 06:40

Physics, 07.01.2022 06:40

Mathematics, 07.01.2022 06:40

Mathematics, 07.01.2022 06:40

Biology, 07.01.2022 06:40


History, 07.01.2022 06:40

English, 07.01.2022 06:40

Health, 07.01.2022 06:50

Mathematics, 07.01.2022 06:50



SAT, 07.01.2022 06:50


Biology, 07.01.2022 06:50


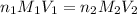
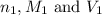 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 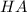
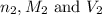 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.