
Chemistry, 07.04.2020 20:18 yournerdybirdyp43oi3
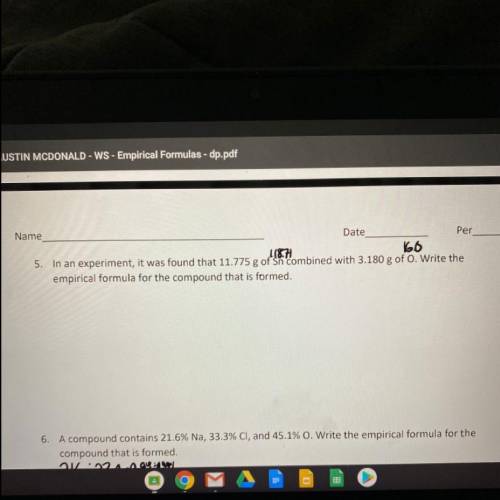
In an experiment, it was found that 11.775 g of Sn combined with 3.180 g of O. Write the empirical formula for the compound that is formed.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

You know the right answer?
In an experiment, it was found that 11.775 g of Sn combined with 3.180 g of O. Write the empirical f...
Questions



History, 30.09.2019 23:40

Business, 30.09.2019 23:40

Mathematics, 30.09.2019 23:40

Computers and Technology, 30.09.2019 23:40


Physics, 30.09.2019 23:40

History, 30.09.2019 23:40

French, 30.09.2019 23:40

Mathematics, 30.09.2019 23:40


Health, 30.09.2019 23:40





Mathematics, 30.09.2019 23:40

Mathematics, 30.09.2019 23:40

Mathematics, 30.09.2019 23:40



