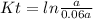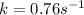Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1
You know the right answer?
The first-order reaction, 2 N2O(g) → 2 N2(g) + O2(g), has a rate constant equal to 0.76 s-1 at 1000...
Questions


Mathematics, 02.12.2021 23:50


Mathematics, 02.12.2021 23:50

Mathematics, 02.12.2021 23:50


Social Studies, 02.12.2021 23:50







History, 02.12.2021 23:50

Business, 02.12.2021 23:50



Medicine, 02.12.2021 23:50

Mathematics, 02.12.2021 23:50

Computers and Technology, 02.12.2021 23:50