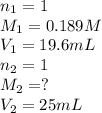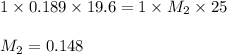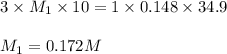Chemistry, 07.04.2020 19:27 KAITLYN007
A 25 ml volume of a sodium hydroxide solution requires 19.6 mL of a 0.189 M HCl acid for neutralization. A 10 mL volume of phosphoric acid solution requires 34.9 mL of the NaOH solution for complete neutralization. Calculate the concentration of the phosphoric acid solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:10
Harvey mixes two liquids. which observation of the new mixture most likely indicates a precipitate is forming?
Answers: 2

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1
You know the right answer?
A 25 ml volume of a sodium hydroxide solution requires 19.6 mL of a 0.189 M HCl acid for neutralizat...
Questions




Physics, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01


Mathematics, 28.09.2020 21:01

Mathematics, 28.09.2020 21:01



Spanish, 28.09.2020 21:01


Mathematics, 28.09.2020 21:01

History, 28.09.2020 21:01


Advanced Placement (AP), 28.09.2020 21:01

Computers and Technology, 28.09.2020 21:01


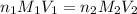
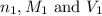 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 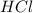
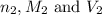 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.