
A sample of nitrogen gas had a volume of 500. mL, a pressure in its closed container of 740 torr, and a temperature of 25 °C. What was the new volume of the gas when the temperature was changed to 50 °C and the new pressure was 760 torr, if the amount of gas does not change?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
The study of witch tree monkeys feed in is part of the science life
Answers: 1

Chemistry, 21.06.2019 18:00
What is the theoretical yield of carbon dioxide? a)0.993 gb)2.98 gc)3.65 gd)8.93 g
Answers: 1


Chemistry, 22.06.2019 19:30
Which liquid (h2o, h2o + soap, or h2o + salt) has the strongest cohesion and adhesion? (need now plz)
Answers: 1
You know the right answer?
A sample of nitrogen gas had a volume of 500. mL, a pressure in its closed container of 740 torr, an...
Questions


History, 08.10.2019 17:00

History, 08.10.2019 17:00


History, 08.10.2019 17:00



English, 08.10.2019 17:00

Computers and Technology, 08.10.2019 17:00





Business, 08.10.2019 17:00

English, 08.10.2019 17:00



Mathematics, 08.10.2019 17:00

Spanish, 08.10.2019 17:00

History, 08.10.2019 17:00

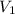 = 500 mL = 0.5 L
= 500 mL = 0.5 L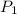 = 740 torr
= 740 torr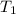 = 25 ° c = 298 K
= 25 ° c = 298 K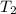 = 50 ° c = 323 K
= 50 ° c = 323 K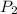 = 760 torr
= 760 torr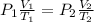
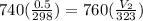
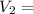 0.526 L = 526 ml
0.526 L = 526 ml

