

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 10:00
Miner's coal distributors does not mine coal itself, nor does it even store or handle the coal. instead, miner's solicits orders for low sulfur coal from other firms, then purchases the required amount from suppliers and directs them to ship the coal to its customers. what is miner's
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

You know the right answer?
A sample of argon gas, Ar(g). is placed in a 3.80 L container at 320 K. The gas pressure is 0.496 at...
Questions



Mathematics, 14.01.2021 20:10

Biology, 14.01.2021 20:10


Mathematics, 14.01.2021 20:10



Mathematics, 14.01.2021 20:10

Mathematics, 14.01.2021 20:10

Social Studies, 14.01.2021 20:10


Computers and Technology, 14.01.2021 20:10

Mathematics, 14.01.2021 20:10

Spanish, 14.01.2021 20:20

Social Studies, 14.01.2021 20:20


Mathematics, 14.01.2021 20:20

Arts, 14.01.2021 20:20

Mathematics, 14.01.2021 20:20

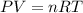 .......(1)
.......(1)


