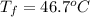Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

You know the right answer?
F 100. grams of liquid water at 100.°C and 200. grams of water at 20.0°C are mixed in an insulated c...
Questions




English, 24.03.2020 01:18


Chemistry, 24.03.2020 01:18


Mathematics, 24.03.2020 01:18


Mathematics, 24.03.2020 01:18


Chemistry, 24.03.2020 01:18





Mathematics, 24.03.2020 01:19

Mathematics, 24.03.2020 01:19

Physics, 24.03.2020 01:19

Mathematics, 24.03.2020 01:19

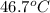
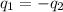
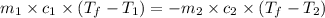
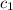 =
= 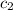 = specific heat of liquid water = Same
= specific heat of liquid water = Same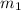 = mass of liquid water = 100 g
= mass of liquid water = 100 g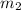 = mass of water = 200 g
= mass of water = 200 g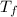 = final temperature of mixture = ?
= final temperature of mixture = ?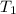 = initial temperature of liquid water =
= initial temperature of liquid water = 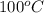
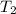 = initial temperature of water =
= initial temperature of water = 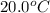
![(100g)\times (T_f-100.0)^oC=-[(200g)\times (T_f-20.0)^oC]](/tpl/images/0586/5833/2dfce.png)