
Chemistry, 07.04.2020 16:59 shanasia76
A 25.0 g sample of an alloy was heated to 100.0 oC and dropped into a beaker containing 90 grams of water at 25.32 oC. The temperature of the water rose to a final value of 27.18 oC. Neglecting heat losses to the room and the heat capacity of the beaker itself, what is the specific heat of the alloy

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Explain how the integumentary system plays a crucial role in the ability to maintain homeoestasis
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2
You know the right answer?
A 25.0 g sample of an alloy was heated to 100.0 oC and dropped into a beaker containing 90 grams of...
Questions


History, 16.10.2020 23:01

History, 16.10.2020 23:01

Mathematics, 16.10.2020 23:01

Chemistry, 16.10.2020 23:01



Mathematics, 16.10.2020 23:01

Mathematics, 16.10.2020 23:01


Geography, 16.10.2020 23:01

Chemistry, 16.10.2020 23:01

Mathematics, 16.10.2020 23:01


Mathematics, 16.10.2020 23:01



History, 16.10.2020 23:01


Mathematics, 16.10.2020 23:01

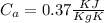
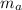 = 25 gm
= 25 gm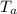 = 100°c = 373 K
= 100°c = 373 K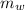 = 90 gm
= 90 gm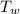 = 25.32 °c = 298.32 K
= 25.32 °c = 298.32 K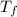 = 27.18 °c = 300.18 K
= 27.18 °c = 300.18 K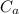 [
[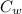 (
(

