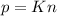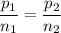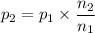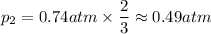A gas exerts a pressure of 0.74 atm in a certain container. Suddenly, a chemical change occurs that consumes half of the molecules originally present and forms two new molecules for every three consumed. Determine the new pressure in the container if the volume of the container and the temperature are unchanged. The reaction that occurs is: 3A(g) —> 2B(g)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Silica, sio2, is formed on silicon as an electrically insulating layer for microelectronic devices. silica is formed when silicon is exposed to o2 gas at an elevated temperature. at 900˚c, it takes 90 minutes for the oxygen to diffuse from the surface to form a 0.06 micron (0.06 x 10-6 m) thick layer of sio2 on
Answers: 1

Chemistry, 21.06.2019 17:00
Noble gases are the most reactive elements on the periodic table. a. true b. false
Answers: 2


Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
A gas exerts a pressure of 0.74 atm in a certain container. Suddenly, a chemical change occurs that...
Questions


History, 20.01.2021 22:20

Advanced Placement (AP), 20.01.2021 22:20

History, 20.01.2021 22:20

Chemistry, 20.01.2021 22:20

Mathematics, 20.01.2021 22:20


Mathematics, 20.01.2021 22:20




Biology, 20.01.2021 22:20


Mathematics, 20.01.2021 22:20

History, 20.01.2021 22:20

Social Studies, 20.01.2021 22:20

Mathematics, 20.01.2021 22:20

Spanish, 20.01.2021 22:20

Mathematics, 20.01.2021 22:20

Business, 20.01.2021 22:20