HX(aq) + H2O(l) → H3O+(aq) + X−(aq)
Based on the equation, HX would be classified as A) an aci...

Chemistry, 07.04.2020 06:38 isabelvaldez123
HX(aq) + H2O(l) → H3O+(aq) + X−(aq)
Based on the equation, HX would be classified as A) an acid, because it accepts a proton
B) a base, because it accepts a proton. C) a base, because it donates a proton D) an acid, because it donates a proton

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2


Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
Questions

History, 17.01.2020 20:31

Mathematics, 17.01.2020 20:31





Social Studies, 17.01.2020 20:31

English, 17.01.2020 20:31

Physics, 17.01.2020 20:31



Biology, 17.01.2020 20:31



Social Studies, 17.01.2020 20:31


Social Studies, 17.01.2020 20:31

Mathematics, 17.01.2020 20:31


Health, 17.01.2020 20:31

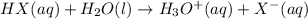
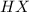 is loosing a proton, thus it is considered as an acid and after losing a proton, it forms
is loosing a proton, thus it is considered as an acid and after losing a proton, it forms 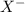 which is a conjugate base.
which is a conjugate base.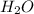 is gaining a proton, thus it is considered as a base and after gaining a proton, it forms
is gaining a proton, thus it is considered as a base and after gaining a proton, it forms 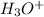 which is a conjugate acid.
which is a conjugate acid.

