Chemistry, 07.04.2020 04:53 granthazenp5e9mj
A 0.623 g sample of a monoprotic acid is dissolved in water and titrated with 0.260 M KOH.
What is the molar mass of the acid if 19.0 mL of the KOH solution is required to neutralize the sample?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1

Chemistry, 23.06.2019 07:00
The image compares the arrangement of electrons in two different neutral atoms. a figure labeled atom q has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has six black spheres. to the left of this figure is another figure labeled atom p. atom p has a shaded sphere at the center of three concentric circles. the innermost circle has two black spheres. the middle circle has seven black spheres. which of the following best explains the position of the two atoms in the periodic table?
Answers: 2

Chemistry, 23.06.2019 07:00
4. glenn andrews recently bought a motorcycle for $3,950. if he had to pay 6% sales tax on the bike, what was the total cost of the motorcycle?
Answers: 1
You know the right answer?
A 0.623 g sample of a monoprotic acid is dissolved in water and titrated with 0.260 M KOH.
Wh...
Wh...
Questions


Mathematics, 12.07.2019 19:40

Biology, 12.07.2019 19:40





Mathematics, 12.07.2019 19:40

Biology, 12.07.2019 19:40

Mathematics, 12.07.2019 19:50

English, 12.07.2019 19:50

Biology, 12.07.2019 19:50


Health, 12.07.2019 19:50

History, 12.07.2019 19:50

Biology, 12.07.2019 19:50

Social Studies, 12.07.2019 19:50



Mathematics, 12.07.2019 19:50

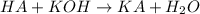
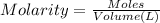
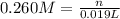
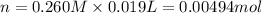
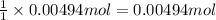 of HA
of HA