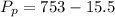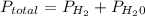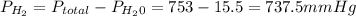Chemistry, 07.04.2020 03:19 blueyish6422
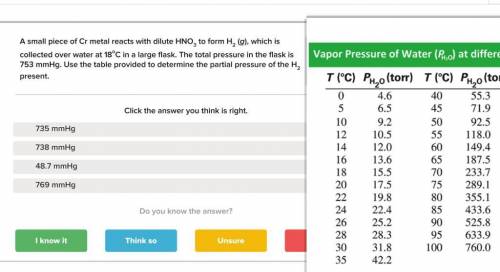
A small piece of Cr metal reacts with dilute HNO3 to form H2 (g), which is collected over water at 18 C in a large flask. The total pressure in the flask is 753 mmHg.
Determine the partial pressure of the H2 present.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:00
Achemist wants to extract copper metal from copper chloride solution. the chemist places 0.50 grams of aluminum foil in a solution containing 0.75 grams of copper (ii) chloride. a single replacement reaction takes place. (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction? a) approximately 0.36 grams, because copper (ii) chloride acts as a limiting reactant b) approximately 1.8 grams, because copper (ii) chloride acts as a limiting reactant c) approximately 0.36 grams, because aluminum acts as a limiting reactant d) approximately 1.8 grams, because aluminum acts as a limiting reactant
Answers: 3

Chemistry, 22.06.2019 21:30
What is another way to determine mass times acceleration?
Answers: 1

Chemistry, 23.06.2019 01:00
Reactions in cells take place at about a. 40°c b. 0° c. 100°c d. 60°c
Answers: 1
You know the right answer?
A small piece of Cr metal reacts with dilute HNO3 to form H2 (g), which is collected over water at 1...
Questions






Arts, 22.08.2019 02:30

Mathematics, 22.08.2019 02:30

Mathematics, 22.08.2019 02:30



Mathematics, 22.08.2019 02:30

Mathematics, 22.08.2019 02:30




English, 22.08.2019 02:30


Mathematics, 22.08.2019 02:30

Mathematics, 22.08.2019 02:30

Mathematics, 22.08.2019 02:30

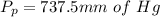
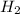 is mathematically represented as
is mathematically represented as 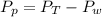
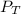 is the total pressure of water with a value of 15.5 mm of Hg
is the total pressure of water with a value of 15.5 mm of Hg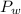 is the partial pressure of water with a value 753 mm of Hg
is the partial pressure of water with a value 753 mm of Hg