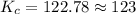Chemistry, 07.04.2020 02:36 maddysmall32
Consider the following reaction: CO(g)+2H2(g) <--> CH3OH(g).
An equilibrium mixture of this reaction at a certain temperature was found to have
[CO]=0.115M, [H2]=0.116M, and [CH3OH]=0.190M. What is the value of the equilibrium constant (Kc) at this temperature?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 21:30
While in europe, if you drive 125 km per day, how much money would you spend on gas in one week if gas costs 1.10 euros per liter and your car’s gas mileage is 32.0 mi/gal? assume that 1 euro=1.26 dollars
Answers: 2

Chemistry, 22.06.2019 22:00
Imagine one batch of soup (batch “a”) is made with 8.19 g/can of salt, according to the recipe, and a second batch of soup (batch “b”) is made with 8.32 g/can of salt. explain which batch would be more resistant to frost damage if it is shipped a great distance in winter and explain why.
Answers: 2
You know the right answer?
Consider the following reaction: CO(g)+2H2(g) <--> CH3OH(g).
An equilibrium mixture of t...
An equilibrium mixture of t...
Questions

Mathematics, 15.04.2021 20:00









Mathematics, 15.04.2021 20:00

Mathematics, 15.04.2021 20:00





Mathematics, 15.04.2021 20:00

Mathematics, 15.04.2021 20:00



Mathematics, 15.04.2021 20:00

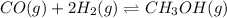
![[CO]=0.115 M,[H_2]=0.116 M](/tpl/images/0585/7114/6fdc2.png)
![[CH_3OH]=0.190 M](/tpl/images/0585/7114/98ee3.png)
![K_c=\frac{[CH_3OH]}{[CO][H_2]^2}](/tpl/images/0585/7114/4cf94.png)