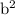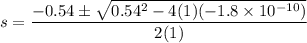Answer the following questions about the solubility of AgCl(s). The value of Ksp for AgCl(s) is 1.8 × 10−10.
Calculate the value of [Ag+] in a saturated solution of AgCl in distilled water.
The concentration of Cl−(aq) in seawater is 0.54 M.
Calculate the molar solubility of AgCl(s) in seawater.
Explain why AgCl(s) is less soluble in seawater than in distilled water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 08:30
Which of the following would have less momentum than a 52 kg cheetah running at 10 m/s?
Answers: 2

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
Answer the following questions about the solubility of AgCl(s). The value of Ksp for AgCl(s) is 1.8...
Questions






Physics, 22.07.2019 12:20




Mathematics, 22.07.2019 12:20


Computers and Technology, 22.07.2019 12:20

English, 22.07.2019 12:20



Biology, 22.07.2019 12:20

Mathematics, 22.07.2019 12:20



Advanced Placement (AP), 22.07.2019 12:20

 M.
M.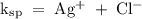
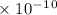 = x
= x  x
x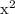

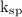 = b
= b