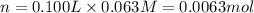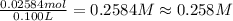Chemistry, 06.04.2020 19:08 cassanovaanthony
Suppose 1.73g of barium nitrate is dissolved in 100.mL of a 63.0mM aqueous solution of sodium chromate.
Calculate the final molarity of nitrate anion in the solution. You can assume the volume of the solution doesn't change when the barium nitrate is dissolved in it. Be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
Suppose 1.73g of barium nitrate is dissolved in 100.mL of a 63.0mM aqueous solution of sodium chroma...
Questions


History, 01.09.2019 09:30

Mathematics, 01.09.2019 09:30

Mathematics, 01.09.2019 09:30

Physics, 01.09.2019 09:30

Mathematics, 01.09.2019 09:30


History, 01.09.2019 09:30


History, 01.09.2019 09:30

History, 01.09.2019 09:30

Physics, 01.09.2019 09:30



Social Studies, 01.09.2019 09:30

History, 01.09.2019 09:30

Mathematics, 01.09.2019 09:30

Physics, 01.09.2019 09:30


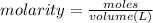
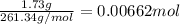
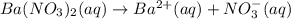
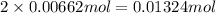 of nitrate ions
of nitrate ions