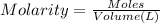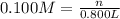Chemistry, 06.04.2020 18:54 devinmoore4664
A chemist must prepare of 800.0 ml potassium hydroxide solution with a pH of 13.00 at 25°.
She will do this in three steps:
Fill a 800 ml volumetric flask about halfway with distilled water.
Weigh out a small amount of solid potassium hydroxide and add it to the flask.
Fill the flask to the mark with distilled water.
Calculate the mass of potassium hydroxide that the chemist must weigh out in the second step. Round your answer to significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Acylinder is filled with 2.00 moles of nitrogen, 3.00 moles of argon and 5.00 moles of helium. if the gas mixture is at stp, what is the partial pressure of the argon
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1
You know the right answer?
A chemist must prepare of 800.0 ml potassium hydroxide solution with a pH of 13.00 at 25°.
Questions




History, 11.07.2019 06:10




English, 11.07.2019 06:10

History, 11.07.2019 06:10

Mathematics, 11.07.2019 06:10

Mathematics, 11.07.2019 06:10

Mathematics, 11.07.2019 06:10

History, 11.07.2019 06:10

Mathematics, 11.07.2019 06:10





Physics, 11.07.2019 06:10

![pOH=-\log[OH^-]](/tpl/images/0584/6258/fe336.png)
![1.00=-\log[OH^-]](/tpl/images/0584/6258/6b8f7.png)
![[OH^-]=10^{-1.00} M=0.100 M](/tpl/images/0584/6258/a9d8c.png)
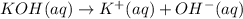
![[KOH]=[OH^-]=[K^+]=0.100 M](/tpl/images/0584/6258/fca0f.png)