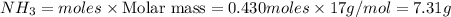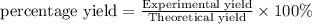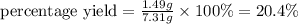Chemistry, 06.04.2020 18:26 gunner20115
The Haber-Bosch process is a very important industrial process. In the Haber-Bosch process, hydrogen gas reacts with nitrogen gas to produce ammonia according to the equation 3H2(g)+N2(g)→2NH3(g)3H2(g)+N2(g)→2N H3(g) The ammonia produced in the Haber-Bosch process has a wide range of uses, from fertilizer to pharmaceuticals. However, the production of ammonia is difficult, resulting in lower yields than those predicted from the chemical equation.1.29g H2 is allowed to react with 9.55g N2, producing 1.49g NH3.
What is the theoretical yield for this reaction under the given conditions?
What is the percent yield for this reaction under the given conditions?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
The Haber-Bosch process is a very important industrial process. In the Haber-Bosch process, hydrogen...
Questions

SAT, 04.10.2021 23:00

SAT, 04.10.2021 23:00

Biology, 04.10.2021 23:00

Mathematics, 04.10.2021 23:00

Mathematics, 04.10.2021 23:00

Mathematics, 04.10.2021 23:00


Mathematics, 04.10.2021 23:00

Biology, 04.10.2021 23:00

SAT, 04.10.2021 23:00

Chemistry, 04.10.2021 23:00


Chemistry, 04.10.2021 23:00

Biology, 04.10.2021 23:00



Mathematics, 04.10.2021 23:00



History, 04.10.2021 23:00

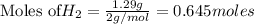
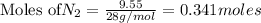
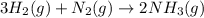
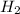 require = 1 mole of
require = 1 mole of 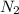
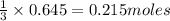 of
of 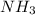
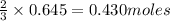 of
of