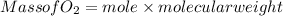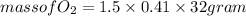Chemistry, 06.04.2020 18:21 roseemariehunter12
Potassium superoxide, KO2, is often used in oxygen masks (such as those used by firefighters) because KO2 reacts with CO2 to release molecular oxygen. Experiments indicate that 2 mol of KO2(s) react with each mole of CO2(g).
(a) The products of the reaction are K2CO3(s) and O2(g). Write a balanced equation for the reaction between KO2(s) and CO2(g).
(b) Indicate the oxidation number for each atom involved in the reaction in part (a). What elements are being oxidized and reduced?
(c) What mass of KO2(s) is needed to consume 18.0 g CO2(g)? What mass of O2(g) is produced during this reaction?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Aroom with dimensions 7.00m×8.00m×2.50m is to be filled with pure oxygen at 22.0∘c and 1.00 atm. the molar mass of oxygen is 32.0 g/mol. how many moles noxygen of oxygen are required to fill the room? what is the mass moxygen of this oxygen?
Answers: 1

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
Potassium superoxide, KO2, is often used in oxygen masks (such as those used by firefighters) becaus...
Questions



Biology, 12.07.2019 16:00


Arts, 12.07.2019 16:00


Mathematics, 12.07.2019 16:00

Chemistry, 12.07.2019 16:00



English, 12.07.2019 16:00

Biology, 12.07.2019 16:00


Biology, 12.07.2019 16:00


Mathematics, 12.07.2019 16:00

Spanish, 12.07.2019 16:00

Mathematics, 12.07.2019 16:00

History, 12.07.2019 16:00

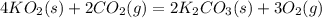
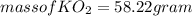 and
and 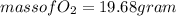
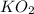 need 2 moles of
need 2 moles of 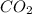 for complete reaction i.e. mole
for complete reaction i.e. mole 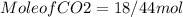
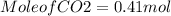
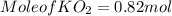
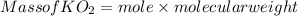
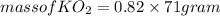
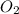 is produced
is produced 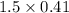 mole of
mole of