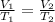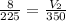Chemistry, 06.04.2020 05:53 cathydaves
8.00 L of sulfur hexafluoride gas are heated from 225K to 350K. If the pressure and number of gas particles do not change, what is the new volume after the temperature increase? 1.00 x 103 L 1.02 x 10-4 L 12.4 L 5.14 L

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Identifying limitations of kinetic-molecular theorya chemist is studying the properties of a gas under various conditions. he observes that when the gas is at room temperature and low pressure, it behaves as an ideal gas. when the gas is cooled to 10 kelvin and is placed under high pressure, however, it deviates significantly from an ideal .
Answers: 1

Chemistry, 22.06.2019 01:30
Asap! how do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2
You know the right answer?
8.00 L of sulfur hexafluoride gas are heated from 225K to 350K. If the pressure and number of gas pa...
Questions

History, 16.12.2020 19:40

Geography, 16.12.2020 19:40

Mathematics, 16.12.2020 19:50


Chemistry, 16.12.2020 19:50

Mathematics, 16.12.2020 19:50

Mathematics, 16.12.2020 19:50

Computers and Technology, 16.12.2020 19:50

Mathematics, 16.12.2020 19:50

Mathematics, 16.12.2020 19:50

Physics, 16.12.2020 19:50



Mathematics, 16.12.2020 19:50



Arts, 16.12.2020 19:50

Spanish, 16.12.2020 19:50

Mathematics, 16.12.2020 19:50

Mathematics, 16.12.2020 19:50