If 25 g of NH3, and 96 g of H2S react according to the following reaction, what is the
maximum...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1


Chemistry, 22.06.2019 21:00
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1
You know the right answer?
Questions




English, 04.04.2020 06:12




Mathematics, 04.04.2020 06:13






Computers and Technology, 04.04.2020 06:13



Mathematics, 04.04.2020 06:14


Geography, 04.04.2020 06:14

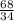 g of (NH₄)₂S
g of (NH₄)₂S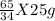 of (NH₄)₂S
of (NH₄)₂S

