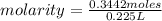Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Does anyone know a lot about how to: - calculate mass of magnesium metal - calculate the actual yield of magnesium oxide - calculate the theoretical yield of mgo - calculate the percent yield of mgo - determine the percent yield of mgo - determine the average percent yield of mgo i had to do an online lab and its asking these questions but i have no idea where to start or how to be able to find these things. i can post the chart of the data from the lab or if you can tell me exactly how i can find each.
Answers: 3

Chemistry, 22.06.2019 01:00
Look at the bean data from days 4–6. use these data to explain how natural selection changed the number of dark red walking beans over time. writing part
Answers: 3

Chemistry, 22.06.2019 01:50
Ase your answer to this question on the information below.hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant.the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen.chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product.nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
What is the molarity of 225 mL of a KNO3 solution containing 34.8 g KNO3? (molar mass = 101.1 g/mol)...
Questions

Mathematics, 03.05.2020 14:08



Biology, 03.05.2020 14:08





Mathematics, 03.05.2020 14:08



English, 03.05.2020 14:08

Mathematics, 03.05.2020 14:08

Mathematics, 03.05.2020 14:08

Mathematics, 03.05.2020 14:08

Mathematics, 03.05.2020 14:08

History, 03.05.2020 14:08


Mathematics, 03.05.2020 14:08

World Languages, 03.05.2020 14:08

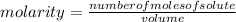
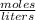 .
.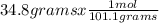 = 0.3442 moles, being 101.1
= 0.3442 moles, being 101.1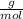 , the molar mass of the compound, that is, the amount of mass that a substance contains in one mole.volume= 225 mL= 0.225 L (being 1000 mL= 1 L)
, the molar mass of the compound, that is, the amount of mass that a substance contains in one mole.volume= 225 mL= 0.225 L (being 1000 mL= 1 L)