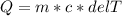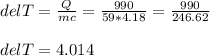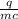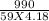JC and D
If 990.J of heat is absorbed by a 59 g sample of water at 21.0 °C, what
will the final temperature of this sample be after absorbing this energy
The specific heat of water is 4.18 J/9g °C). *
25 degrees Celsius
O
35 degrees Celsius
o
2.5 degrees Celsius

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:00
The molality of calcium chloride (cacl2) in an aqueous solution is 2.46 m. what is mole fraction of the solute?
Answers: 3

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
JC and D
If 990.J of heat is absorbed by a 59 g sample of water at 21.0 °C, what
will th...
If 990.J of heat is absorbed by a 59 g sample of water at 21.0 °C, what
will th...
Questions

History, 16.10.2020 20:01






Mathematics, 16.10.2020 20:01

English, 16.10.2020 20:01


Mathematics, 16.10.2020 20:01


Mathematics, 16.10.2020 20:01


Computers and Technology, 16.10.2020 20:01

Biology, 16.10.2020 20:01




Chemistry, 16.10.2020 20:01

English, 16.10.2020 20:01