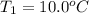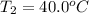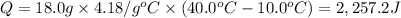Chemistry, 05.04.2020 03:16 meganwintergirl
Calculate the energy needed to raise the temperature of 18.0g of water from 10.0C to 40.0C. The specific heat of water is 4.18 J/gC. *

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 23:00
What is the formula of the ionic compound composed of calcium cations and chloride anions
Answers: 1

Chemistry, 22.06.2019 23:30
If it is an isoelectronic series select true, if not select false. o2-, s2-, se2-, te2- na+, k+, rb+, cs+ n3-, p3-, as3-, sb3- ag, cd+, sn3+, sb4+ f-, cl-, br-, i- f-, ne, na+, mg2+ s2-, s, s6+
Answers: 1

Chemistry, 23.06.2019 04:20
The equation below shows a chemical reaction. a + b + heat —> c + d according to the law of conservation of energy, which statement is true? a. the reactants absorb heat because they have less energy than the products. b. the products release heat because they have more energy than the reactants. c. the reactants generate heat because they have more energy than the products. d. the products require heat to form because they have less energy than the reactants.
Answers: 1

Chemistry, 23.06.2019 10:30
When a wire with a current is placed in a magnetic field, electrical energy is transformed into mechanical energy select the best answer from the choices provided t f
Answers: 2
You know the right answer?
Calculate the energy needed to raise the temperature of 18.0g of water from 10.0C to 40.0C. The spec...
Questions


Social Studies, 18.11.2019 05:31



Mathematics, 18.11.2019 05:31



Chemistry, 18.11.2019 05:31


History, 18.11.2019 05:31

Physics, 18.11.2019 05:31

Mathematics, 18.11.2019 05:31

History, 18.11.2019 05:31

Chemistry, 18.11.2019 05:31


Mathematics, 18.11.2019 05:31

Business, 18.11.2019 05:31


Geography, 18.11.2019 05:31

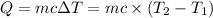
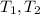 : Initial and final temperature of the substance
: Initial and final temperature of the substance