Chemistry, 04.04.2020 20:50 heavendl13
A chemist reacts magnesium with hydrochloric acid and collects 57.48 mL of the hydrogen gas that is produced by water displacement. If the lab temperature is 24 C and the atmospheric pressure is 742.1 mm Hg, how many grams of hydrogen are produced? Water vapor pressure is 22.4 mm Hg at 24 C.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 06:00
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1
You know the right answer?
A chemist reacts magnesium with hydrochloric acid and collects 57.48 mL of the hydrogen gas that is...
Questions

Mathematics, 04.09.2021 17:40


Mathematics, 04.09.2021 17:40

Social Studies, 04.09.2021 17:40


World Languages, 04.09.2021 17:40

English, 04.09.2021 17:40


Social Studies, 04.09.2021 17:40

Mathematics, 04.09.2021 17:40

German, 04.09.2021 17:40

Mathematics, 04.09.2021 17:40



Mathematics, 04.09.2021 17:50


English, 04.09.2021 17:50

Biology, 04.09.2021 17:50

Mathematics, 04.09.2021 17:50

English, 04.09.2021 17:50

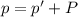

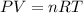 ( Ideal gas equation)
( Ideal gas equation)