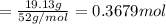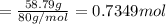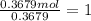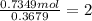Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2

Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3

Chemistry, 23.06.2019 03:30
Ahelium balloon contains 16.9 l of helium at stp. how many atoms of helium are in the balloon
Answers: 1
You know the right answer?
A 19.13 gram sample of chromium is heated in the presence of excess bromine. A metal bromide is form...
Questions

Computers and Technology, 15.06.2021 19:00

Chemistry, 15.06.2021 19:00


Mathematics, 15.06.2021 19:00

Mathematics, 15.06.2021 19:00

Business, 15.06.2021 19:00

Mathematics, 15.06.2021 19:00


Mathematics, 15.06.2021 19:00

Mathematics, 15.06.2021 19:00

Mathematics, 15.06.2021 19:00


Mathematics, 15.06.2021 19:00


Mathematics, 15.06.2021 19:00


Mathematics, 15.06.2021 19:00

Mathematics, 15.06.2021 19:00


Mathematics, 15.06.2021 19:00

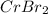 .
.