
Chemistry, 04.04.2020 11:05 ellaemtagedeane
When iron(III) oxide reacts with aluminum, aluminum oxide and iron are produced. The balanced equation for this reaction is:
Fe2O3 (s) + 2Al (s) > Al2O3 (s) + 2Fe (s)
If 6 moles of aluminum react:
i. The reaction consumes moles of iron(III) oxide.
ii. The reaction produces moles of aluminum oxide and moles of iron.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
When iron(III) oxide reacts with aluminum, aluminum oxide and iron are produced. The balanced equati...
Questions


Mathematics, 02.04.2020 21:12


Mathematics, 02.04.2020 21:12


Mathematics, 02.04.2020 21:12


English, 02.04.2020 21:12

History, 02.04.2020 21:12











Biology, 02.04.2020 21:13

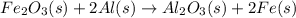
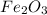
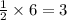 moles of
moles of 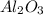
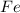
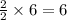 moles of
moles of 

