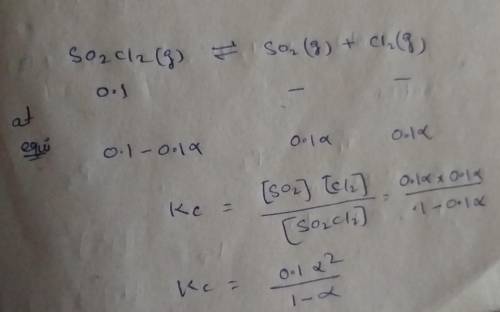Chemistry, 04.04.2020 09:40 kolbehoneyman
Consider the reaction below. Initially the concentration of SO2Cl2 is 0.1000 M. Solve for the equilibrium concentration of SO2Cl2((g). SO2Cl2(g) ←⎯⎯→ SO2(g) + Cl2(g) Kc = 2.99 x 10-7 at 227 °C

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 23.06.2019 14:00
Which is not true regarding reaction rates? (2 points) catalysts are not used up in the reaction. catalysts speed up reactions by lowering the activation energy. reaction rates decrease as the concentration of reactants decrease. during reactions, concentrations of all reactants decrease at the same rate.
Answers: 1

Chemistry, 23.06.2019 17:00
Помогите решить задачу с оформлением дано найт решение пожалуйста? ? номер у прошу
Answers: 1

You know the right answer?
Consider the reaction below. Initially the concentration of SO2Cl2 is 0.1000 M. Solve for the equili...
Questions



Mathematics, 18.06.2020 22:57





Mathematics, 18.06.2020 22:57



Mathematics, 18.06.2020 22:57







English, 18.06.2020 22:57

Mathematics, 18.06.2020 22:57

Mathematics, 18.06.2020 22:57

![[SO_2Cl_2] = 0.09983 M](/tpl/images/0582/0550/0c89a.png)
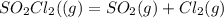
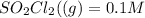
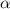
![[SO_2Cl_2] = 0.1-0.1\alpha](/tpl/images/0582/0550/ed240.png)
![[SO_2] = 0.1\alpha](/tpl/images/0582/0550/97c2e.png)
![[Cl_2] = 0.1\alpha](/tpl/images/0582/0550/13ee9.png)
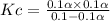
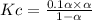

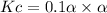
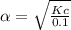
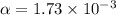
![[SO_2Cl_2] = 0.1-0.1\alpha = 0.1-0.1\times 0.00173](/tpl/images/0582/0550/6431a.png)