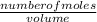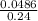Chemistry, 04.04.2020 08:31 Boondiddy9
Suppose that you have 135 mL of a buffer that is 0.360 M in both propanoic acid ( C 2 H 5 COOH ) and its conjugate base ( C 2 H 5 COO − ) . Calculate the maximum volume of 0.240 M HCl that can be added to the buffer before its buffering capacity is lost.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
How is 0.00235 expressed in proper scientific notation? a. 2.35 × 10-3 b. 0.235 × 10-2 c. 2.35 d. 2.35 × 103
Answers: 1

Chemistry, 22.06.2019 05:20
Identify and describe the three ways that mutations affect organisms.
Answers: 1

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 14:50
Complete the following statements to describe solids, liquids, and gases. select the correct answer from each drop-down menu. a solid a definite volume and a definite shape. a liquid a definite volume and a definite shape. a gas a definite volume and a definite shape
Answers: 1
You know the right answer?
Suppose that you have 135 mL of a buffer that is 0.360 M in both propanoic acid ( C 2 H 5 COOH ) and...
Questions


World Languages, 11.09.2021 05:20

Arts, 11.09.2021 05:20

Mathematics, 11.09.2021 05:20

English, 11.09.2021 05:20


Mathematics, 11.09.2021 05:20

Social Studies, 11.09.2021 05:20

Mathematics, 11.09.2021 05:20

History, 11.09.2021 05:20




Health, 11.09.2021 05:20


Mathematics, 11.09.2021 05:20

Mathematics, 11.09.2021 05:20

Social Studies, 11.09.2021 05:20

Mathematics, 11.09.2021 05:20

Chemistry, 11.09.2021 05:20