
Chemistry, 04.04.2020 02:33 nate102201
Potassium hydroxide is very soluble in water, resulting in extremely basic solutions. A 121g sample KOH is dissolved in water at 25∘C to make up 100.0mL of solution. The molar mass of KOH is 56.11gmol. What is the pH of the solution at 25.0∘C?

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2


Chemistry, 23.06.2019 07:00
Under what conditions will a gas be most likely to exhibit the ideal gas properties predicted by the ideal gas law? 1)high pressures and high temperature, because particles are forced closer together with higher kinetic energy, so intermolecular forces of attraction are weaker 2)high pressure and low temperature, because particles are forced closer together and moving slower, so the volume of the particles is less significant 3) low pressure and high temperature, because particles are spread farther apart and moving faster, so the intermolecular forces of attraction are weaker 4)low pressure and low temperature, because particles are spread farther apart with lower kinetic energy, so the volume of the particles is less significant
Answers: 2

Chemistry, 23.06.2019 21:10
What might the student have done that caused this error? list all possible causes
Answers: 1
You know the right answer?
Potassium hydroxide is very soluble in water, resulting in extremely basic solutions. A 121g sample...
Questions

Mathematics, 03.04.2021 04:20



Mathematics, 03.04.2021 04:20



History, 03.04.2021 04:20




Mathematics, 03.04.2021 04:20

Mathematics, 03.04.2021 04:20


Biology, 03.04.2021 04:20

Social Studies, 03.04.2021 04:20




Biology, 03.04.2021 04:20

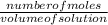
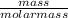 =
= 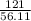 = 2.16mole
= 2.16mole 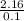 = 21.6moldm⁻³
= 21.6moldm⁻³

