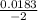Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2


Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1
You know the right answer?
H2SO4 is a strong acid because the first proton ionizes 100%. The Ka of the second proton is 1.1x10-...
Questions

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Chemistry, 17.09.2020 14:01

Physics, 17.09.2020 14:01

English, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

English, 17.09.2020 14:01

Social Studies, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Biology, 17.09.2020 14:01

Social Studies, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

English, 17.09.2020 14:01

Mathematics, 17.09.2020 14:01

![\frac{[SO42-] [H3O+]}{[HSO4-]}](/tpl/images/0580/8225/cd1fe.png)
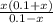
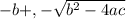 / 2a
/ 2a![\sqrt[-(-o.111)]{(-0.111)^2 - 4(-1) (0.0011) }](/tpl/images/0580/8225/19ba8.png) / 2(-1)
/ 2(-1)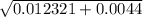 / -2
/ -2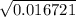 / -2
/ -2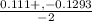
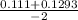 , x =
, x = 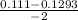
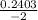 , x =
, x =