Hc2h3o2(aq) + ba(oh)2(aq) + > h2o(l) + ba(c2h3o2)(aq)
balance this equation and determine...


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 21.06.2019 18:30
In an oxidation-reduction reaction, oxidation is what happens when a reactant
Answers: 1

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

You know the right answer?
Questions




History, 25.08.2019 14:30

History, 25.08.2019 14:30



Mathematics, 25.08.2019 14:30




World Languages, 25.08.2019 14:30

Mathematics, 25.08.2019 14:30

History, 25.08.2019 14:30



English, 25.08.2019 14:30


English, 25.08.2019 14:30

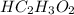
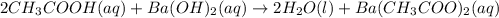
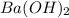
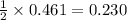 moles of
moles of 


