
Chemistry, 30.08.2019 13:30 gracie0818
After 1.00 ml of water is completely vaporized to gas, how many milliliters of vapor are produced at standard temperature and pressure? (density of water is 1.00 g/ml; molar volume of any gas at stp is 22.4 l/

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 04:00
Acontainer holds 35.8 moles of gas under 10.0 atm of pressure at 70.0 c. what is the volume of the container?
Answers: 2

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1
You know the right answer?
After 1.00 ml of water is completely vaporized to gas, how many milliliters of vapor are produced at...
Questions

Mathematics, 30.06.2019 13:50

Biology, 30.06.2019 13:50

English, 30.06.2019 13:50

English, 30.06.2019 13:50

Mathematics, 30.06.2019 13:50




Mathematics, 30.06.2019 14:00

Spanish, 30.06.2019 14:00

Social Studies, 30.06.2019 14:00

Mathematics, 30.06.2019 14:00

Mathematics, 30.06.2019 14:00

History, 30.06.2019 14:00

Social Studies, 30.06.2019 14:00


Social Studies, 30.06.2019 14:00

Physics, 30.06.2019 14:00

Geography, 30.06.2019 14:00

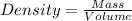
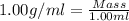
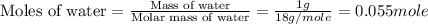
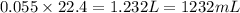 of volume (Conversion factor: 1 L = 1000 mL)
of volume (Conversion factor: 1 L = 1000 mL)

