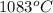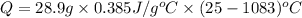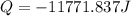Chemistry, 29.12.2019 18:31 jeffhuffle17
Calculate the energy released when a 28.9 gram piece of paper is cooled from its melting point of 1083 degrees celsius to 25.0 degrees celsius. the specific heat of copper is .385 j/g celsius.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1

Chemistry, 22.06.2019 14:30
The valence of aluminum is +3, and the valence of the chlorine is -1. the formula fir the aluminum chloride is correctly written as
Answers: 2

Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1
You know the right answer?
Calculate the energy released when a 28.9 gram piece of paper is cooled from its melting point of 10...
Questions


Mathematics, 22.02.2021 17:20

Mathematics, 22.02.2021 17:20


Physics, 22.02.2021 17:20






Mathematics, 22.02.2021 17:20


Mathematics, 22.02.2021 17:20



Health, 22.02.2021 17:20

Mathematics, 22.02.2021 17:20


Chemistry, 22.02.2021 17:20

Social Studies, 22.02.2021 17:20

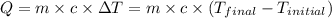
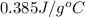
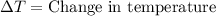
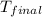 = final temperature =
= final temperature = 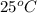
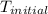 = initial temperature =
= initial temperature =