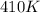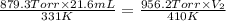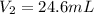Chemistry, 03.04.2020 04:31 wolfiewolffromsketch
A 21.6 ml ball has a pressure of 879.3 torr at 331 K. If the temperatire spikes 410 K and the pressure increases to 956.2 torr what is the volume of the bal

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 06:30
The following reaction shows sodium carbonate reacting with calcium hydroxide. na2co3 + ca(oh)2 → naoh + caco3 how many grams of naoh are produced from 20.0 grams of na2co3? (molar mass of na = 22.989 g/mol, c = 12.01 g/mol, o = 15.999 g/mol, ca = 40.078 g/mol, h = 1.008 g/mol) 12.2 grams 15.1 grams 24.4 grams 30.2 grams
Answers: 2

You know the right answer?
A 21.6 ml ball has a pressure of 879.3 torr at 331 K. If the temperatire spikes 410 K and the pressu...
Questions

Mathematics, 22.03.2021 19:50








Biology, 22.03.2021 19:50




Computers and Technology, 22.03.2021 19:50

Mathematics, 22.03.2021 19:50


Computers and Technology, 22.03.2021 19:50




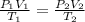
 = initial pressure inside a ball = 879.3 Torr
= initial pressure inside a ball = 879.3 Torr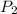 = final pressure inside a ball = 956.2 Torr
= final pressure inside a ball = 956.2 Torr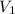 = initial volume of ball =
= initial volume of ball = 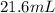
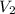 = final volume of gas = ?
= final volume of gas = ?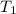 = initial temperature of gas =
= initial temperature of gas = 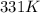
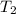 = final temperature of gas =
= final temperature of gas =